There are currently more than 2,500 lawsuits that have been filed on
behalf of individuals throughout the United States who suffered a Levaquin tendon rupture. Of those cases, more than 1512 are pending in New Jersey
state court and about 1000 are pending in federal court, where the litigation
has been centralized for pretrial preceedings as part of an MDL, or
multidistrict litigation, in the U.S. District Court for the District of
Minnesota before U.S. District Judge John Tunheim. A recent conference in
Minnesota was held to review many subjects pertaining to Levaquin litigation. According to a prior Wall Street Journal report, one of
the latest Levaquin lawsuits filed in a New Jersey state court on behalf of
three plaintiffs from around the U.S. charged
that Johnson & Johnson and
Ortho-McNeil represented Levaquin as a safe antibiotic despite its known
association with tendon damage.
The conference in Minnesota
went over issues such as the number of pending cases and anticipated cases. So
far, over 1,300 cases have been filed in New Jersey and over 1,800 in Illinois by patients suffering from Levaquin side effects.
Another issue that was gone over was the confirmation of the date of the first
New Jersey trial set to go forward on August 29th of this year. Lawsuits,
which were recently reported as having begun to circulate through the courts,
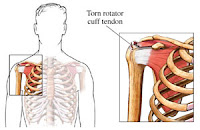
allege that Levaquin has caused Achilles’ tendon ruptures and other damage.
Just over a year ago, federal regulators ordered that Levaquin and similar
antibiotics bear Black Box warnings about their association with serious tendon
injuries.
Levaquin (levofloxacin) is an antibiotic
which is prescribed to prevent bacteria from rapidly reproducing, causing
infection. It is part of a class of antibiotics known as
fluoroquinolones. Although many of the reported cases of tendon ruptures
have been associated with the use of Levaquin, the side effects have also been
seen with the use of other antibiotics which are part of this class, including
Cipro. The FDA has received reports of at least
336 individuals who experienced a tendon rupture after using Cipro, Levaquin or
one of the fluoroquinolone antibiotics. The most common tendon rupture involved
the Achilles tendon. In addition, hundreds of other people have experienced
tendonitis and other tendon disorders.
No comments:
Post a Comment