When Levaquin entered
the market, warnings about tendon Levaquin
side effects were required on all
fluoroquinolone labels, but were buried in a long list of potential adverse
reactions, the plaintiffs claim. In addition, the warnings did not advise that
tendon injury was tripled with fluoroquinolone use in people older than 60 and
in those who are on corticosteroid therapy, according to the complaints. In
fact, Levaquin manufacturers marketed the drug toward the elderly, especially
those with upper respiratory infections who were likely to be chronic
corticosteroid users, the suits state. "More disturbingly, Defendants'
promotional campaign was themed on Levaquin's excellent safety profile and
failed to disclose the risks of tendon injury," the complaints say.
Johnson and Johnson and its subsidiary, Ortho-McNeil, are named as defendants
because they test and manufacture Levaquin.
The
FDA has received reports of hundreds of individuals who experienced a Levaquin
tendon rupture
after using Levaquin or one of the other fluoroquinolones. As a result of the
inadequate warnings currently provided, the consumer advocacy group, Public
Citizen, filed a Levaquin lawsuit in January 2008 as a result of the
FDA’s failure to act on a petition they filed in 2006, calling for more
detailed information about the risk of Levaquin
tendon ruptures to be added to
the warning label. In addition to this,






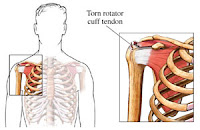





 Levaquin is a 3rd-generation fluoroquinolone antibiotic, manufactured by Ortho-McNeil, a subsidiary of Johnson & Johnson. Doctors prescribe Levaquin as an antibiotic to treat bacterial infections in many different parts of the body. It does not work for viral infections (for example, the common cold). While patients taking Levaquin have reported a variety of minor side-effects, common with any medication, the most serious Levaquin side effect reported has been an increased risk of Achilles tendonitis among current and former patients. Several studies published in accredited medical journals have cited case studies in which individuals who have taken Levaquin have been adversely affected by levofloxacin-induced tendonopathy and tendon rupture.
Levaquin is a 3rd-generation fluoroquinolone antibiotic, manufactured by Ortho-McNeil, a subsidiary of Johnson & Johnson. Doctors prescribe Levaquin as an antibiotic to treat bacterial infections in many different parts of the body. It does not work for viral infections (for example, the common cold). While patients taking Levaquin have reported a variety of minor side-effects, common with any medication, the most serious Levaquin side effect reported has been an increased risk of Achilles tendonitis among current and former patients. Several studies published in accredited medical journals have cited case studies in which individuals who have taken Levaquin have been adversely affected by levofloxacin-induced tendonopathy and tendon rupture. The Minnesota federal judge overseeing the Levaquin multidistrict litigation allowed punitive damages in the first trial after finding that plaintiff John Schedin presented reasonable evidence that defendant Ortho-McNeil-Janssen Pharmaceuticals Inc. knew or disregarded research that the antibiotic could cause tendon injuries, tried to prevent European regulatory action that would affect the drug's reputation and "manipulated" a study "to produce a commercially favorable result," according to an order that was unsealed May 12.
The Minnesota federal judge overseeing the Levaquin multidistrict litigation allowed punitive damages in the first trial after finding that plaintiff John Schedin presented reasonable evidence that defendant Ortho-McNeil-Janssen Pharmaceuticals Inc. knew or disregarded research that the antibiotic could cause tendon injuries, tried to prevent European regulatory action that would affect the drug's reputation and "manipulated" a study "to produce a commercially favorable result," according to an order that was unsealed May 12. 
