A Levaquin tendon rupture lawsuit has been filed by Diane K. Eccles of Oregon, claiming that she suffered from Levaquin tendon ruptures in her shoulder, following using the medication for six months. Although there has been information about the potential risk of tendon damage on the drug’s label since it was first approved by the FDA in 1996, plaintiffs alleged that those warnings were insufficient and that the drug maker actively attempted to downplay the risk for years. Levaquin is the best-selling antibiotic in the U.S., pulling in about $1.5 billion last year. It is now also available as a generic from other drug makers. The most common Levaquin Lawsuit involves ruptures in the achilles tendon, though a growing number of cases are involving tears in the shoulder.
The FDA, in 2008, required an upgraded warning on tendon damage posed by Levaquin and similar drugs. In 2006, Public Citizen filed a petition with the FDA, requesting that stronger warnings be issued regarding Levaquin tendon rupture side effects. In January 2008, Public Citizen filed a lawsuit against the FDA in an attempt to compel the agency to order Levaquin’s maker to include a warning on the Levaquin label regarding the risk of tendon ruptures and other tendon injuries. Finally, in July 2008, the FDA issued a health alert notifying the manufacturers of certain antibiotics, including Levaquin’s maker, of the need to add a boxed warning to the prescribing information about the increased risk of developing tendonitis and tendon rupture in patients taking fluoroquinolones.
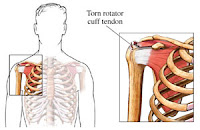
The tendon most frequently associated to the Levaquin induced ruptures is the Achilles tendon, however Levaquin has also been linked to tendon ruptures in the rotator cuff (shoulder), the biceps, the hand, and the thumb. So far, two Levaquin lawsuits of a reported 2,500 pending claims have made their way to court. One was found in favor of the plaintiff while the other was found in favor of Johnson & Johnson. The first lawsuit resulted in a jury awarded $1.8 million to a man who alleged he ruptured both Achilles tendons. In June 2011, however, a jury found in favor of Johnson & Johnson in a Levaquin lawsuit, after determining that the company properly warned about the risks associated with the antibiotic.